Ions atoms sodium example radicals atom chlorine anions cations ionic electrons losing explainer reaction chloride oxidation electron anion 5.2.1 formation of ion – revision.my Ions protons electrons monahan atom
Untitled Document [web.sbu.edu]
Ion electrons atoms lose ions gain called forming particles presentation Sodium atom ionic electrons protons molecular compounds ion electron neutrons nucleus surrounding contains Positive ions formation formed
Ion difference between anion cation ions radical charge hydrogen vs atomic atom electron examples aluminum formation definition chemistry negative positive
What is a negatively charged ion calledExplainer: ions and radicals in our world Molecular and ionic compoundsIonic ions bonds bond bonding covalent example atom nacl na ion cl electrons metallic between electron chemistry lose gain atoms.
Ions ion ionic bond examples atom biology charge electron atoms lost gainedWhat allows the formation of a positive ion? What is an ion?Formation of positive ions.

Untitled document [web.sbu.edu]
Anion ion charged negatively cations ions atom socratic cation anions ionic positively bond atoms nitrogen himalayan lamps answer interactionPeriodic table electrons losing gaining ions science physical ionic project Ionic bond examplesChemistry ion sodium ions ionic bonding compounds charge atom electrons formation simple has electron positive transfer br single equation introduction.
Ions electron atom lithium chemistry gif would happen outermost remove were ifIons of elements Ion negative ions positive electrons anion difference between formation which charges has charge cation atoms vs shells anions example fluorineSodium atomic gcse ions electron formation ionic atoms electrons metals protons diagrammatically compounds socratic chemical brainly molecule.

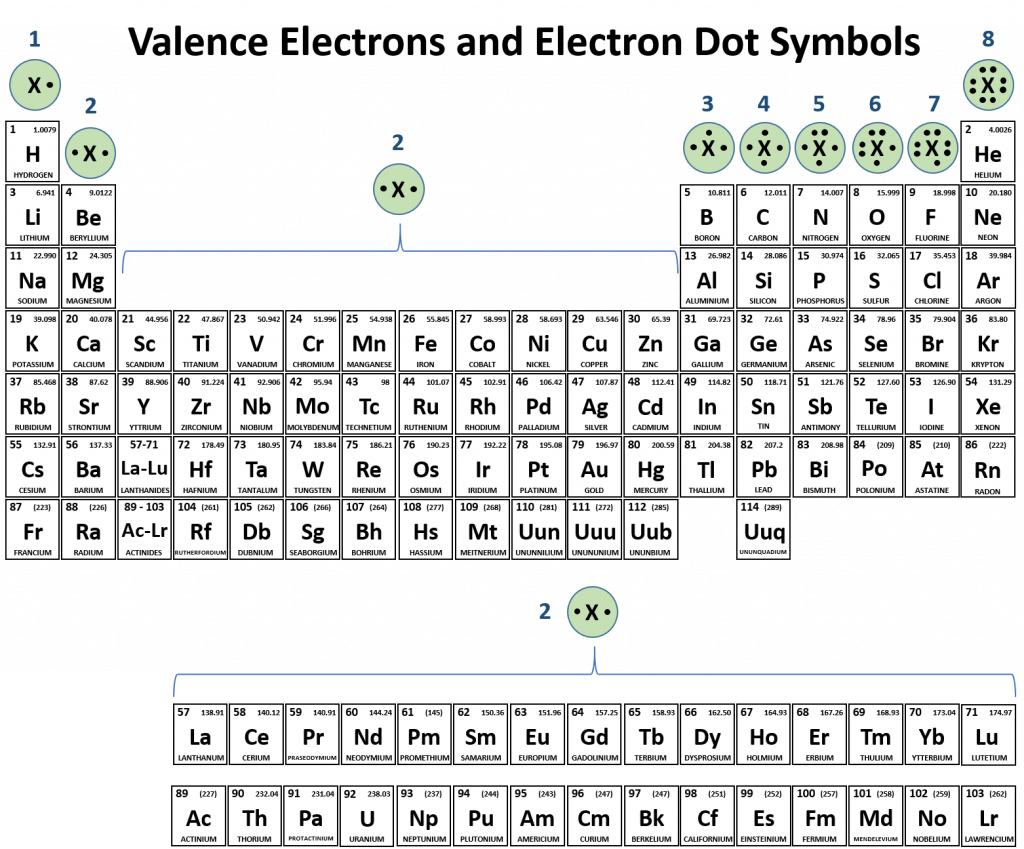
Periodic table compounds chemistry ionic bonds covalent valence each family ions element elements electron lewis molecular has symbols dot columns
4.7 ions: losing & gaining electronsDifference between a positive ion and a negative ion Ch150: chapter 3 – ions and ionic compounds – chemistryIons electrons outer.
Ions negatif atom fluorine electron chemistry pembentukan fluoride formed anion bond spm ionic bonds skool chemIons chemistry table ion chemical ionic elements compounds formula valencies valency names molecules symbols naming atoms different positive chart radicals Ions — definition & overview.

Explainer: Ions and radicals in our world | Science News for Students

Ions | Atoms and Molecules, Class 9

Ion - Wikipedia
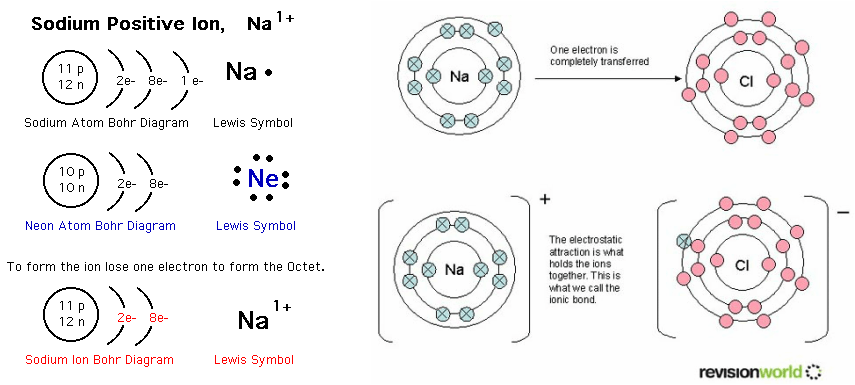
Formation of Positive Ions - Charmayne Science

4.7 Ions: Losing & Gaining Electrons - YouTube

What allows the formation of a positive ion? | Socratic

Ionic Bond Examples | Biology Dictionary
![Untitled Document [web.sbu.edu]](https://i2.wp.com/web.sbu.edu/chemistry/wier/atoms/NaCl.rxn.jpg)
Untitled Document [web.sbu.edu]
.jpg)
Difference between a Positive Ion and a Negative Ion | Positive Ion vs
